


The Approach - Quality by Design
Risk is an inevitable part of a clinical trial but it is not always unavoidable! At DiagnoSearch, Risk Management principles based on ISO 31010 guidelines form the backbone of every clinical trial. A “Quality by Design” approach in the truest sense is applied by building quality into every step of the Risk Management process. This involves risk planning, risk assessment at the outset, lifecycle approach and use of risk assessment tools to set off triggers and alerts, risk detection and timely action or mitigation.
The Process - Identify, Analyze, Evaluate, Control!
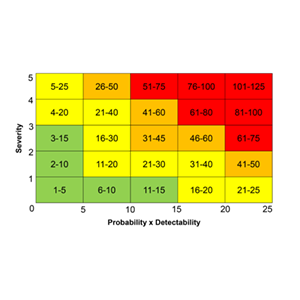 At DiagnoSearch Risk Management is a very systematic process beginning with risk assessment, risk analysis, risk evaluation by experienced Risk Managers who then work towards risk control (risk reduction or mitigation). DiagnoSearch leverages its rich clinical trial experience across various therapeutic areas and all phases of clinical development. A gamut of information based on the experience, historical data and informed opinions form the basis for identification of potential risks right at the outset of a project. In addition, every trial comes with unanticipated risks which cannot be forecast in the beginning; however, these can be detected by trained Risk Managers through a systematic risk detection approach. The complex and vast data generated during a clinical trial is reviewed in a structured manner, as it emerges, by a cross-functional team of experts. With global emphasis on a Risk Based Monitoring approach to focus on critical data elements and processes, a high powered monitoring practiced to ensure a quality conduct of clinical trial and data documentation. The data is examined from a subject, site, project and program level perspective and is scrutinized for concerns and trends. The issues, adverse trends or observations are deliberated further to verify if they qualify as potential risks. These risks are further analyzed employing Risk Management tools and risk scores are assigned to evaluate the detectability of risks, probability of occurrence and the severity. The risks with high scores are escalated to all the stake holders immediately and the process of risk control is initiated. Our Risk Managers brainstorm to apply risk reduction methods to either bring the risk score to an acceptable level of severity or to mitigate it completely!
At DiagnoSearch Risk Management is a very systematic process beginning with risk assessment, risk analysis, risk evaluation by experienced Risk Managers who then work towards risk control (risk reduction or mitigation). DiagnoSearch leverages its rich clinical trial experience across various therapeutic areas and all phases of clinical development. A gamut of information based on the experience, historical data and informed opinions form the basis for identification of potential risks right at the outset of a project. In addition, every trial comes with unanticipated risks which cannot be forecast in the beginning; however, these can be detected by trained Risk Managers through a systematic risk detection approach. The complex and vast data generated during a clinical trial is reviewed in a structured manner, as it emerges, by a cross-functional team of experts. With global emphasis on a Risk Based Monitoring approach to focus on critical data elements and processes, a high powered monitoring practiced to ensure a quality conduct of clinical trial and data documentation. The data is examined from a subject, site, project and program level perspective and is scrutinized for concerns and trends. The issues, adverse trends or observations are deliberated further to verify if they qualify as potential risks. These risks are further analyzed employing Risk Management tools and risk scores are assigned to evaluate the detectability of risks, probability of occurrence and the severity. The risks with high scores are escalated to all the stake holders immediately and the process of risk control is initiated. Our Risk Managers brainstorm to apply risk reduction methods to either bring the risk score to an acceptable level of severity or to mitigate it completely!
An Interdisciplinary Affair, throughout the Life Cycle of a Project!
Risk Management is initiated right from the start by interdisciplinary teams comprising of medical experts, study monitors, project managers, biostatisticians, data reviewers and regulatory affairs personnel. A rigorous data surveillance, insightful data analytics and high powered monitoring throughout the life cycle by all relevant stakeholders helps to identify and address potential issues before they turn into full blown problems.
The IT Advantage
‘Risk Management’ became a buzzword in clinical research a few years ago and now it is an indispensable aspect of every clinical trial. To effectively accomplish the Risk Management process, DiagnoSearch envisioned to leverage Information Technology to build automated algorithms into processes. The result is Wide-Angle-Data, DiagnoSearch’s Central, Integrated Data Review Platform that uses Risk Management principles based on ISO 31010 guidelines as the central theme. Data migration, cross-functional seamless data integration, centralized review, multiple data review algorithms, powerful data visualization, real-time data analytics and continuous risk updates are some of thekey features of the platform. Wide-Angle-Data houses an inbuilt risk engine which helps identify early risk triggers. Additionally, Wide-Angle-Data’s ability to seamlessly integrate data on a single platform aids a consolidated review by all key study personnel. The reviewers can examine concerns, raise issues, post them for peer review, perform root cause analysis, recommend mitigation strategies and narrow down the potential risks which get updated into the statistical risk engine on a real-time basis.
The Core Components of the Risk Management System at DiagnoSearch include:
